Updated: October 9th, 2025
Overview
The National Health and Nutrition Examination Survey (NHANES) is a program designed to assess the health and nutritional status of the U.S. population. Initiated under the National Health Survey Act of 1956, the program initially focused on statistically measuring the extent, distribution, and impact of chronic illnesses, such as cardiovascular disease and diabetes, and disabilities through three National Health Examination Surveys (NHES). In the 1970s, the program expanded to include research on diet-disease relationships, where it adopted the name NHANES 3,4.
NHANES has significantly impacted public health by supplying essential data for developing public health strategies and policies, particularly through its detailed dietary research, which has informed national nutritional guidelines and initiatives to combat chronic diseases 2. NHANES is representative of the U.S. population, utilizing complex, multistage, highly-stratified clusters to randomly select participants, ensuring generalizability to the U.S. population as a whole. The survey design often includes oversampling of underrepresented groups, such as ethnic minorities and vulnerable populations, to enhance research accuracy 4–6.
There have been three NHES cycles, five NHANES installments, and four ancillary studies, each using different sampling methods and sometimes focusing on specific populations or health outcomes 7–11. The number of participants enrolled varies between each iteration, typically ranging from 5,000 to 10,000 participants per year 5,6,12–19.
The types of data collected include:
Demographic Data: Basic demographics like age, gender, race, socioeconomic status, family characteristics, and geographic location information such as state and urbanicity.
Health and Medical Examinations: Medical history and records, physical and dental examinations, vision and hearing tests, X-rays, and anthropometric measurements such as height, weight, and body mass index.
Laboratory Work: Blood, urine, and other biological specimens are collected to test for nutritional biomarkers, environmental exposures, and indicators of infectious and chronic diseases.
Psychosocial and Behavioral Data: Psychological evaluations including measures of mental health and distress, behavioral assessments covering smoking, alcohol, and drug use, and physical activity and fitness measures.
Questionnaire Results: Participants provide self-reported information on a variety of health-related topics, including medical history, health conditions, and behaviors like smoking and physical activity.
Dietary Data: Nutritional status assessments, including dietary intake, nutrient composition, dietary frequency, and 24-hour food consumption recall.
Special Focus Variables: Detailed examinations for chronic conditions including, but not limited to, child growth and development metrics, epidemiologic follow-ups, and specific tests and variables based on subpopulation-focused surveys such as the Hispanic Health and Nutrition Examination Survey (HHANES) 7–10,20.
Because NHANES collects data from different individuals in two-year cycles, it is a cross-sectional survey rather than a longitudinal one. The complex sampling design ensures nationally representative results when analyzed with the provided sample weights for each installment.
Researchers interested in combining cross-sectional iterations for longitudinal research should carefully review the recommendations provided in the NHANES Questionnaires, Datasets, and Related Documentation on the CDC landing page for each program 11. Additional support can be found in:
National Health and Nutrition Examination Survey: 1999–2022 Survey Content Brochure 21
NHANES Survey Methods and Analytic Guidelines, which consolidates some of the program’s documentation 22
2017–March 2020 Prepandemic File: Sample Design, Estimation, and Analytic Guidelines for additional details 23
Gaining Access
Do I Qualify?
All individuals seeking to use the data for statistical analysis and reporting purposes may access and download it.
Typical Timeline
There are no time constraints for accessing these data.
Step-by-Step Guide
No specific steps are required. Researchers can navigate to the NHANES Questionnaires, Datasets, and Related Documentation page, find the desired NHANES program installment link, and download the data from there 11.
Be aware that combining two different installments or biannual Continuous NHANES datasets requires specific considerations. Refer to the relevant data documentation, the list of helpful resources provided earlier, or any of the webinars and tutorials available for further guidance on handling survey design and protocol differences.
Do I Qualify?
Individuals seeking to use data for statistical analysis and reporting may submit research proposals to the NCHS Research Data Center (RDC) through the Standard Application Process (SAP) via the ResearchDataGov (RDG) portal 24,25.
Applicants must fulfill criteria during application review and, in some cases, after approval. The four core criteria below are summarized from the RDG User Guide 26. If additional post-approval steps are required, data-owning agencies contact applicants directly to initiate those processes.
Identification: Researchers verify identity, job title, organizational affiliation, and skill level. Some agencies require U.S. citizenship confirmation.
Training: Some agencies require post-approval training on data use, management, confidentiality, and cybersecurity.
Agreements: Researchers sign agreements such as non-disclosure or data use agreements. Some data sources require security plans outlining data protection methods.
Investigation: Researchers undergo background checks.
How Is My Application Assessed?
All applications submitted through SAP are evaluated against the same criteria regardless of the agency or unit, unless required by law or regulation. Full criteria are available in the RDG User Guide and summarized below 26:
Statistical Purpose: Data is used solely for statistical purposes, not to identify individuals or businesses, nor for law enforcement, legal cases, or regulatory actions.
Allowed Use: Researchers plan to use data in compliance with applicable rules and restrictions.
Statistical Disclosure Limitation: Researchers must employ techniques that protect individual, organizational, or business privacy.
Demonstrated Need: Researchers must demonstrate that confidential data is necessary for project goals and that publicly available data is insufficient.
Feasibility: Researchers can achieve project goals with requested data. This is evaluated in three ways:
Project Design: Detailed planned methods and how technical and logistical needs will be met.
Agency or Unit Support: Confirmation that agencies can provide space, technical assistance, logistics, and data preparation.
Applicant Ability: Applicants possess the knowledge, skills, and ability to execute the project.
- Maintaining Public Trust: Projects are expected to maintain public trust and confidence in the agency or unit.
Additionally, the NCHS Review Committee evaluates studies for well-defined public health research questions, benefits to data-providing agencies, and appropriateness of planned outputs (papers, articles, presentations) 27.
NCHS does not evaluate studies for scientific merit or relevance (substantive, methodological, theoretical, or policy). Study output release is not guaranteed due to privacy concerns and application compliance requirements.
Typical Timeline
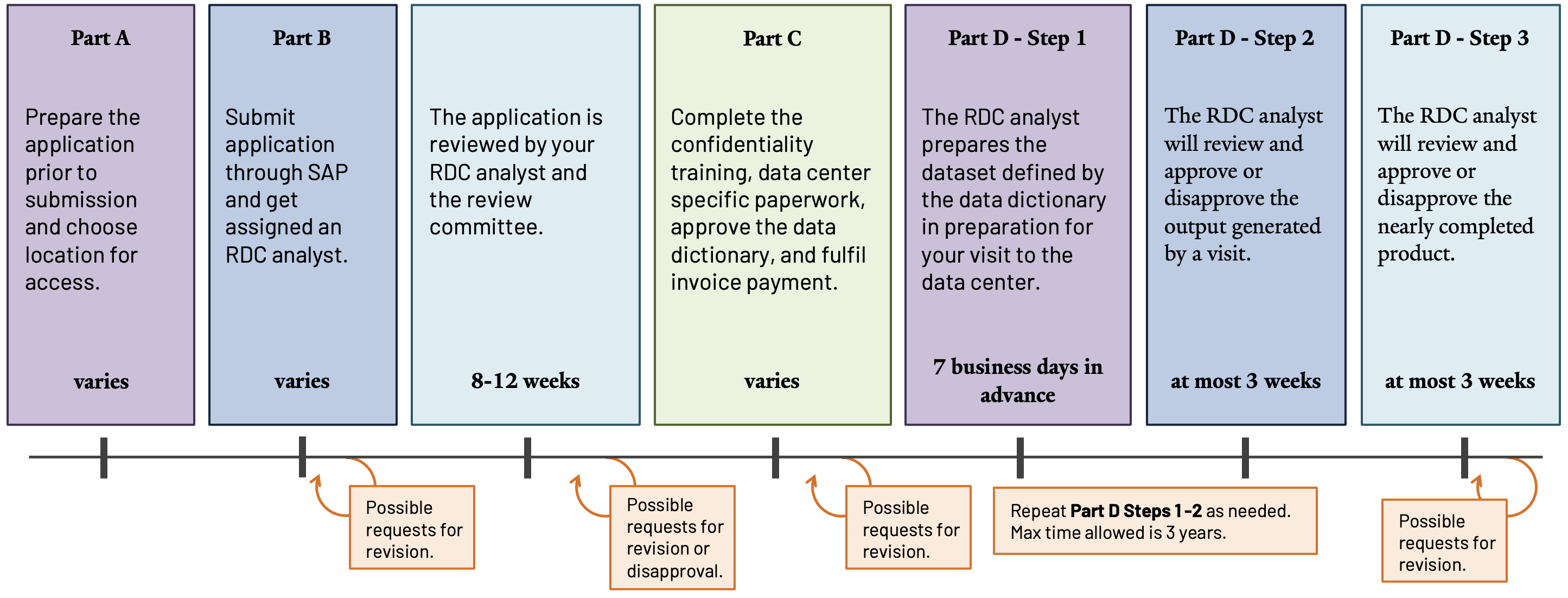
Upon receiving applications, assigned RDC analysts and the review committee—comprised of data system representatives and the confidentiality officer—assess applications for approval, disapproval, or revision requests. If denied, researchers receive justification for the decision and may request appeals through SAP 28.
Studies may require amendments to approved applications. Amendment approvals typically take up to four weeks, but complicated requests may require eight to twelve weeks.
Step-by-Step Guide
a. Prepare Your Application Before Completing the Form
The RDG portal provides access to applications for data from 13 agencies and 3 units within the Federal Statistical System (FSS), including NCHS. Each agency may have additional application requirements. While the RDG User Guide and RDC Application Process page contain detailed information, researchers are encouraged to contact relevant agencies before submitting applications 24,26.
Example applications, data dictionaries, and complete details are available on the RDC Application Process page 24. This summary provides an outline and directs researchers to relevant information sources. Contact the RDC at rdca@cdc.gov for further assistance 26.
Confirm the study requires restricted data access and that FSS data will meet research needs. Contact all relevant agencies or units to verify that required data can be combined, whether restricted-use, publicly available, or non-FSS.
Select a preferred access location and confirm eligibility. Non-U.S. citizens should contact the intended facility before writing proposals to confirm eligibility for data access. Refer to the Data Distribution Centers section for more details.
Data access methods and locations may vary, especially if the data comes from different agencies or units within the FSS.
- The NCHS requires additional documents as *.pdf or *.docx and additional concerns to be addressed beyond the base SAP application. Before starting the application, it is recommended to prepare the following:
A data dictionary listing all requested variables from publicly available, restricted-use, and non-NCHS data sources, which can largely be created using SAP. Refer to the Providing the Public Use and Non-NCHS Data page for additional guidelines on preparing applications with these data types 29.
A description of the research methodology and a list of supporting references.
A detailed description of the intended data product (e.g., paper, journal article, or presentation).
A timeline for managing the project within a three-year period.
An explanation of how the project will benefit NCHS or other data-providing agencies for the Agency Benefits section of the application.
b. Complete the SAP Application Form
- In SAP, search for and select the datasets needed for the study and add them to the checkout basket. Any data added to the same basket will be included in a single application.
Additional data can be added to the application if needed after submission.
When ready to proceed, open the basket and select Start Application. It is recommended that the principal investigator (PI) or co-principal investigator (co-PI) create the application, as only they will be able to make edits after submission.
In the application, the researcher will need to provide their information, a description of the project that demonstrates the need for restricted-use data, and upload the required documents as *.pdf or *.docx. The SAP application and some agency- or unit-specific requirements can be found in the RDG User Guide 26.
Submit the application for review.
c. Review the Committee’s Decision and Finalize the Application
Upon receiving the application, the RDC director will assign the group an RDC analyst who will work with them throughout the entire process of applying for data, accessing the data, and finalizing the output of their results 27. The RDC analyst will:
- Facilitate application review and accept NCHS-required confidentiality paperwork.
- Accept payments incurred by data center access.
- Create datasets by compiling data specified in data dictionaries and linking using designated variables. Analytical datasets are provided during data center visits.
- Review results for disclosure risk and provide them once analysis is completed at data centers.
Researchers with approved applications must complete the following steps to prepare for data access and utilization.
Discuss the committee’s approval with the assigned RDC analyst and address any revision requests as needed.
Provide the RDC analyst with approved data dictionaries, public-use and non-NCHS data, descriptions of desired data linkages with intended final formats, and clearly defined derived variables, including arithmetic code or algorithms.
Complete the Confidentiality and Disclosure training and forms, then provide them to the RDC analyst. Additional forms, documents, and tools are available on the RDC Reference Materials page 30,31.
When invoiced, pay fees incurred by the request and applicable to data access at the intended location and frequency. Complete details are in Fees and Invoicing 32.
Amendments for project changes are possible but require additional approval prior to implementation. Common reasons for an amendment include, but are not limited to, changes in the research team, requests for additional variables, new methods, or requests for additional types of output 24. However, if the scope or research question changes, a new application is required instead.
Contact the RDC directly at rdca@cdc.gov for further guidance.
d. Accessing the Data and Publication Expectations
- After completing the steps in Part C, the RDC analyst will prepare the dataset. Once it is ready, schedule an appointment to access the data at the chosen RDC location. Schedule appointments at least one week in advance.
Different data centers have different access procedures and may incur additional fees depending on frequency of data access. Refer to the Data Distribution Centers section and associated links for additional guidance.
- Upon completion of analysis, submit an output request to the RDC analyst for review and approval. Full details about requirements and expectations are on the Output Policies and Procedures page 33.
NCHS does not guarantee that study-generated output will be released due to concerns related to, but not limited to, privacy and alignment with the approved application.
- When output is nearly complete, send it to the RDC analyst for review before submitting for publication or distribution. Full details about requirements and expectations are on the Publishing Guidelines page 34.
Data Distribution Centers
NCHS data is available at two types of data centers: the Census Bureau’s Federal Statistical Research Data Centers (FSRDC) and the NCHS Research Data Centers (RDCs). Additional details about preparing to access data at one of these sites can be found on the RDC Location of Access page or their respective subpages: FSRDC and NCHS RDC 24,35–37.
Submit an electronic copy of any notes or reference materials needed to the RDC analyst prior to visiting a center. Hard copies of these materials are not allowed. Electronic communication devices, such as phones, pagers, and laptops, are also not permitted in the RDC.
Different software products or add-ons can be requested, though not all requests will be approved. Be aware that requesting additional software accommodations may delay project approval. Contact the specific RDC where data will be accessed for further guidance.
Federal Statistical Research Data Centers
Researchers must be affiliated with a university or agency to qualify for FSRDC access. They must also meet physical and information security requirements, including obtaining Census Bureau Special Sworn Status (SSS) and passing a background investigation. Non-U.S. citizens are generally encouraged to use FSRDCs.
Researchers are assigned both an NCHS RDC analyst and an FSRDC administrator from the location they intend to visit. The NCHS RDC analyst roles were described previously under Part C of the Step-by-Step Guide. The FSRDC (Census RDC) administrator will:
- Be available to answer questions pertaining to SSS, access and entry to a FSRDC location, software availability, and additional Census fees.
- They may be available to help develop the application.
- Transfer output to the NCHS after researchers complete their analysis at a data center. The RDC analyst will review the output for disclosure risk and provide researchers with the results.
If accessing an FSRDC, the data must be transferred from NCHS after completing all requirements outlined by the assigned RDC analyst seven days prior to the intended visit. These additional steps incur extra costs beyond the NCHS RDC data access fee and may delay data access.
- In-person: Census Bureau facilities at partner institutions.
- Remote access: A secure Virtual Desktop Interface (VDI) may be available.
- Software Available: Anaconda, Gurobi, Intel Composer, Knittro, MADD, Mathematica, NATLAB, OpenGeoda, R and Rstudio, SAS, Stat/Transfer, Stata, Stata-MP, SUDAAN, and Tomlab.
NCHS Research Data Centers
- In-person: Facilities located in Hyattsville, MD, Atlanta, GA, and Rockville, MD require appointments scheduled at least one week in advance.
- Remote access: Not available due to computers being disconnected from the internet.
- Software Available: Microsoft Office products, R, SAS, Stata, Python, SPSS (v. 19), and SAS-callable SUDAAN.
Required Documentation
When accessing the data, researchers must provide the following documentation in addition to an approved application:
- Proof of identification, such as a REAL ID
- Curriculum vitae (CV) for each applicant
- For student projects, an agreement form completed by the student’s advisor 38
- Permission to use proprietary data
- Table shells for requested output
- Data dictionary listing all necessary variables (restricted-use, public-use, and external) for the research project (refer to agency dataset data dictionaries for available variables)
Relevant Links
NHANES Questionnaires, Datasets, and Related Documentation: Find links to all installments or ancillary studies and their associated program methods and documentation 11.
NCHS Data User Agreement: Find the data use limitations and expectations set by NCHS for using their publicly available data 39.
Confidentiality and Disclosure: Find study participant confidentiality training, documentation, forms, and manual 31. These trainings and forms must be completed following approval of the study proposal by the research committee.
Application Process: Find the complete step-by-step guide for applying for restricted-use data from the RDC 24. Key information is summarized under the Gaining Access - Restricted Access tab.
RDC Reference Materials: Find restricted-use data proposals, example applications, confidentiality and disclosure documents, rules, policies, and data protection toolkits 30.
NHANES Biospecimen Program: Find the request forms for DNA, serum, plasma, and urine samples as well as genetic data produced from the NHANES biospeciments 40.
NHANES Webinars: Find additional information and resources for program partners and data users, including updates and survey strategies 41.
NHANES Tutorials: Find help understanding sample design, variance estimation, interpreting documentation with example analyses, software tips, and sample code 42.
What NHANES Data Have Achieved: Find a listing of the public health research inspired by NHANES data and the policy changes it has influenced 2.
Publications
This section presents a selection of PubMed articles that utilize the dataset and are authored by individuals affiliated with the Yale University. These articles are provided to inspire researchers and students to use the data in their own work.
-
United States Long-Term Trends in Adult BMI (1959-2018): Unraveling the Roots of the Obesity Epidemic.
Julia Banas, Acree McDowell Cook, Karina Raygoza-Cortez, Daniel Davila, Melinda L Irwin, Leah M Ferrucci, Debbie L Humphries
International journal of environmental research and public health 2024 Jan 9 doi: 10.3390/ijerph21010073
PMID: 38248537 -
A multivariate joint model to adjust for random measurement error while handling skewness and correlation in dietary data in an epidemiologic study of mortality.
George O Agogo, Leacky Muchene, Benedict Orindi, Terrence E Murphy, Henry Mwambi, Heather G Allore
Annals of epidemiology 2023 Mar 25 doi: 10.1016/j.annepidem.2023.03.007
PMID: 36972757 -
Clinical Characteristics of Adults at Risk of Medicaid Disenrollment Due to HR 1 Work Requirements.
Ashwin K Chetty, Joseph S Ross, Alissa S Chen
JAMA doi: 10.1001/jama.2025.16533
PMID: 41032309 -
Nonexercise machine learning models for maximal oxygen uptake prediction in national population surveys.
Yuntian Liu, Jeph Herrin, Chenxi Huang, Rohan Khera, Lovedeep Singh Dhingra, Weilai Dong, Bobak J Mortazavi, Harlan M Krumholz, Yuan Lu
Journal of the American Medical Informatics Association : JAMIA doi: 10.1093/jamia/ocad035
PMID: 36905605 -
Association Between Rental Assistance Programs and Hemoglobin A1c Levels Among US Adults.
Andrew Fenelon, Kasia J Lipska, Whitney Denary, Kim M Blankenship, Penelope Schlesinger, Denise Esserman, Danya E Keene
JAMA network open 2022 Jul 1 doi: 10.1001/jamanetworkopen.2022.22385
PMID: 35857325 -
Trends and Key Factors Associated With Racial and Ethnic Differences in Life’s Essential 8 Scores.
Huanhuan Yang, Chenxi Huang, Mitsuaki Sawano, Jeph Herrin, Kamil F Faridi, Zhihui Li, Erica Spatz, Harlan M Krumholz, Yuan Lu
JAMA network open 2025 Jun 2 doi: 10.1001/jamanetworkopen.2025.16663
PMID: 40531529 -
Sex-Specific Risk Factors Associated With First Acute Myocardial Infarction in Young Adults.
Yuan Lu, Shu-Xia Li, Yuntian Liu, Fatima Rodriguez, Karol E Watson, Rachel P Dreyer, Rohan Khera, Karthik Murugiah, Gail D’Onofrio, Erica S Spatz, Khurram Nasir, Frederick A Masoudi, Harlan M Krumholz
JAMA network open 2022 May 2 doi: 10.1001/jamanetworkopen.2022.9953
PMID: 35503221