Updated: January 12th, 2026
Overview
The National Vital Statistics System (NVSS) is operated by the Division of Vital Statistics within the National Center for Health Statistics (NCHS) 1. National collection of vital statistics (births, deaths, fetal deaths, marriages, divorces, and annulments) began with the U.S. Census Bureau in 1900 and transferred to NCHS in 1946 under the Public Health Service Act (42 USC 242k). Since then, NCHS has collected and disseminated annual vital records while providing recommendations to ensure uniform laws, forms, procedures, and statistical methodology 2.
Data is compiled from 57 registration areas: all 50 U.S. states, the District of Columbia, New York City, American Samoa, Guam, Northern Mariana Islands, Puerto Rico, and the Virgin Islands. These jurisdictions operate independently and register vital events within their boundaries 1. To establish standardization among these diverse areas, NCHS issues periodic guidelines and certificates that reflect changing social conditions and evolving user demands. These standards are updated approximately every 10 years to meet current needs 2.
Because data is sourced from all these jurisdictions, the NVSS is representative of the U.S. population. However, users should be aware that some datasets may have under- or overestimated occurrences of specific outcomes, depending on the data collection methods applied that year 2,3.
The types of data collected include 4–6:
Demographic Data: Basic demographics such as age, gender, race, socioeconomic status, family characteristics, and geographic location information.
Marriage and Divorce Data: Statistics on marriages, divorces, and annulments, including characteristics of the individuals (e.g., age, race, previous marital status) and geographic data.
Birth Data: Information on birth certificates, including birth rates, characteristics of births (e.g., place of birth, birth weight, gestational age), and maternal and paternal demographics.
Death and Mortality Data: Data from death certificates, including mortality rates, causes of death, underlying and contributing factors, decedent demographics, and detailed data on deaths by specific causes, including chronic diseases, infectious diseases, and external causes such as accidents and violence.
Fetal Death Data: Data on fetal deaths, including information on gestational age, birth weight, maternal characteristics, and causes of fetal death.
Drug Overdose Deaths: Data includes cause-of-death information, specific drugs involved, ICD-10 codes, and updates from ongoing investigations reported by medical examiners and coroners.
NVSS has also conducted specialized programs and data linking efforts addressing specific concerns that can be analyzed using vital statistics. These include 7–10:
Linked Birth/Infant Death Data: Contains linked data from birth certificates and subsequent infant death certificates to analyze factors associated with infant mortality.
Matched Multiple Birth Data Set: Includes information on multiple births, such as twins and triplets, matched to assess outcomes and characteristics of these births.
National Maternal and Infant Health Survey: Collects data on maternal and infant health, including prenatal care, pregnancy complications, and infant health outcomes.
National Mortality Followback Survey: Compiles detailed information on decedents, including healthcare utilization and circumstances around their deaths, to analyze mortality patterns and contributing factors.
Gaining Access
Do I Qualify?
All individuals seeking to use the data for statistical analysis and reporting purposes may access and download it.
Typical Timeline
There are no time constraints for accessing these data.
Step-by-Step Guide
Researchers can access publicly available data from two webpages: the Vital Statistics Online Data Portal and the CDC’s Wide-ranging ONline Data for Epidemiologic Research (WONDER) Application Programming Interface (API) 3,4.
Do I Qualify?
Two versions of restricted data are available, each offering different information levels, access requirements, and researcher obligations. National micro-data can be requested directly from NVSS by following the process outlined on the Restricted-Use Vital Statistics Data webpage, with key features summarized below3.
This restricted data level provides researchers access to:
Additional demographic and geographic details suppressed since 2005
Event timing to the nearest month, and sometimes week (exact dates are not provided)
Access to values otherwise suppressed due to small counts
NCHS changed its maternal mortality coding, publication, and release methods in 2018. A separate restricted-use data file containing cause-of-death codes is available through an NCHS Research Data Center (RDC) to help researchers reconcile these changes with previously released data from 2003-2017 11.
Researchers seeking to use this data must only be 3,12:
Conducting research studying health and factors affecting health
Demonstrating capacity to advance scientific knowledge about health-related issues
Requestors CANNOT use the data or request access if they plan to:
Assess health-related issues for a single state (researchers should contact the applicable state directly for such data)
Conduct analysis requiring geographic data more specific than what is described in the previously linked data dictionaries
Use exact dates in their research
Access data remotely from outside the U.S./territories or lead projects as overseas Principal Investigators without U.S. physical presence and institutional affiliation
Link the data to other datasets that may re-identify participants
Use the data for commercial or resale purposes
How Is My Application Assessed?
Successful applicants will meet the previously mentioned expectations and provide sufficient evidence of their ability to secure accessed data and adhere to cybersecurity requirements. Researchers seeking this type of restricted data are encouraged to thoroughly review the Restricted-Use Vital Statistics Data webpage and the Project Review Form to understand expectations before pursuing access 12.
Key considerations include, but are not limited to:
Suppress counts below 10 (including totals or rates based on these counts) when preparing results for distribution
Store and access data on secure computer systems of the affiliated organization or institution, with limited exceptions possible if proper cybersecurity measures are implemented
Provide a description of the institution’s data protection procedures, storage systems, and designated personnel responsible for Division of Vital Statistics (DVS) restricted data security
If sharing data with researchers from multiple institutions, provide data protection procedures and identify the responsible party at each institution
Researchers are NOT allowed to store data on portable devices or commercial cloud platforms (e.g., Dropbox, Google Drive, Microsoft Azure, Amazon Web Services) 12.
Typical Timeline
Allow 4-6 weeks for application processing, with initial review taking 2-4 weeks. Researchers have access to data for 2 years following submission of a signed NCHS Data Use Agreement, with the possibility of applying for an extension. Requests to extend access or add new data years will require an amended application based on the original 3,12.
Step-by-Step Guide
Confirm that your study requires restricted data access and that restricted micro-data (not RDC data) will meet your research needs. Review the Descriptions of Data Files/Record Layouts to ensure they are sufficient for your research project 3.
Complete the Project Review Form, also available on the same page. Include all required and supporting documents as PDFs with your application 12.
Students submitting as principal investigators must provide a letter of support from their primary mentor or advisor on institutional letterhead. Additional details are available in the Project Review Form 12.
- An authorized institutional official (not the PI, co-investigators, or analysts) must sign the Data Use Agreement on behalf of the applicant’s organization. Each institution that will house copies of the files requires a separate agreement 12.
Following NCHS project approval, direct all inquiries about file delivery status, access issues with specific files or variables, personnel modifications, and extensions for existing file access (without additional data years) to dvsdatarequests@cdc.gov 12.
Researchers must either return the micro-data and compressed files to NCHS or securely destroy all data files upon completion of the approved project period, unless they have requested and received approval for renewal 3.
Do I Qualify?
Two versions of restricted data are available, each offering different information levels, access requirements, and researcher obligations. The complete datasets can be requested through the NCHS Research Data Center (RDC) by following the Standard Application Process (SAP) via the ResearchDataGov (RDG) portal 13,14.
This restricted data level provides researchers access to:
All demographic and geographic details, including sub-county level information
Exact dates and timing for highly sensitive variables
The ability to gain approval for linkage studies using data available through the RDC
Applicants must satisfy criteria both during application review and, when necessary, after approval. Researchers seeking data for statistical analysis and reporting typically qualify for access. The RDG User Guide 15 outlines four core criteria, summarized below. If post-approval steps are needed, data-owning agencies will contact applicants directly to begin those processes.
Identification: Researchers verify identity, job title, organizational affiliation, and skill level. Some agencies require U.S. citizenship confirmation.
Training: Some agencies require post-approval training on data use, management, confidentiality, and cybersecurity.
Agreements: Researchers sign agreements such as non-disclosure or data use agreements. Some data sources require security plans outlining data protection methods.
Investigation: Researchers undergo background checks.
How Is My Application Assessed?
All applications submitted through SAP are evaluated against the same criteria regardless of the agency or unit, unless required by law or regulation. Full criteria are available in the RDG User Guide and summarized below 15:
Statistical Purpose: Data is used solely for statistical purposes, not to identify individuals or businesses, nor for law enforcement, legal cases, or regulatory actions.
Allowed Use: Researchers plan to use data in compliance with applicable rules and restrictions.
Statistical Disclosure Limitation: Researchers must employ techniques that protect individual, organizational, or business privacy.
Demonstrated Need: Researchers must demonstrate that confidential data is necessary for project goals and that publicly available data is insufficient.
Feasibility: Researchers can achieve project goals with requested data. This is evaluated in three ways:
Project Design: Detailed planned methods and how technical and logistical needs will be met.
Agency or Unit Support: Confirmation that agencies can provide space, technical assistance, logistics, and data preparation.
Applicant Ability: Applicants possess the knowledge, skills, and ability to execute the project.
- Maintaining Public Trust: Projects are expected to maintain public trust and confidence in the agency or unit.
Additionally, the NCHS Review Committee evaluates studies for well-defined public health research questions, benefits to data-providing agencies, and appropriateness of planned outputs (papers, articles, presentations) 16.
NCHS does not evaluate studies for scientific merit or relevance (substantive, methodological, theoretical, or policy). Study output release is not guaranteed due to privacy concerns and application compliance requirements.
Typical Timeline
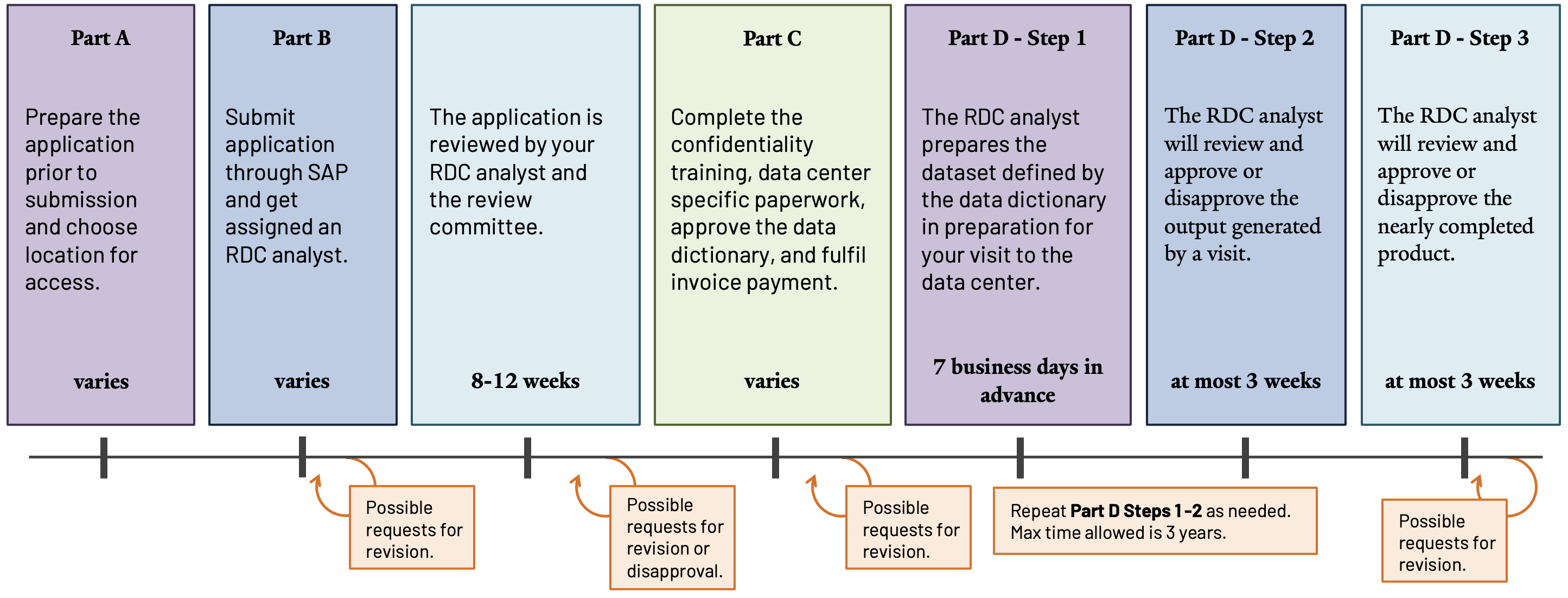
Upon receiving applications, assigned RDC analysts and the review committee—comprised of data system representatives and the confidentiality officer—assess applications for approval, disapproval, or revision requests. If denied, researchers receive justification for the decision and may request appeals through SAP 17.
Studies may require amendments to approved applications. Amendment approvals typically take up to four weeks, but complicated requests may require eight to twelve weeks.
Step-by-Step Guide
a. Prepare Your Application Before Completing the Form
The RDG portal provides access to applications for data from 13 agencies and 3 units within the Federal Statistical System (FSS), including NCHS. Each agency may have additional application requirements. While the RDG User Guide and RDC Application Process page contain detailed information, researchers are encouraged to contact relevant agencies before submitting applications 13,15.
Example applications, data dictionaries, and complete details are available on the RDC Application Process page 13. This summary provides an outline and directs researchers to relevant information sources. Contact the RDC at rdca@cdc.gov for further assistance 15.
Confirm the study requires restricted data access and that FSS data will meet research needs. Contact all relevant agencies or units to verify that required data can be combined, whether restricted-use, publicly available, or non-FSS.
Select a preferred access location and confirm eligibility. Non-U.S. citizens should contact the intended facility before writing proposals to confirm eligibility for data access. Refer to the Data Distribution Centers section for more details.
Data access methods and locations may vary, especially if the data comes from different agencies or units within the FSS.
- The NCHS requires additional documents as *.pdf or *.docx and additional concerns to be addressed beyond the base SAP application. Before starting the application, it is recommended to prepare the following:
A data dictionary listing all requested variables from publicly available, restricted-use, and non-NCHS data sources, which can largely be created using SAP. Refer to the Providing the Public Use and Non-NCHS Data page for additional guidelines on preparing applications with these data types 18.
A description of the research methodology and a list of supporting references.
A detailed description of the intended data product (e.g., paper, journal article, or presentation).
A timeline for managing the project within a three-year period.
An explanation of how the project will benefit NCHS or other data-providing agencies for the Agency Benefits section of the application.
b. Complete the SAP Application Form
- In SAP, search for and select the datasets needed for the study and add them to the checkout basket. Any data added to the same basket will be included in a single application.
Additional data can be added to the application if needed after submission.
When ready to proceed, open the basket and select Start Application. It is recommended that the principal investigator (PI) or co-principal investigator (co-PI) create the application, as only they will be able to make edits after submission.
In the application, the researcher will need to provide their information, a description of the project that demonstrates the need for restricted-use data, and upload the required documents as *.pdf or *.docx. The SAP application and some agency- or unit-specific requirements can be found in the RDG User Guide 15.
Submit the application for review.
c. Review the Committee’s Decision and Finalize the Application
Upon receiving the application, the RDC director will assign the group an RDC analyst who will work with them throughout the entire process of applying for data, accessing the data, and finalizing the output of their results 16. The RDC analyst will:
- Facilitate application review and accept NCHS-required confidentiality paperwork.
- Accept payments incurred by data center access.
- Create datasets by compiling data specified in data dictionaries and linking using designated variables. Analytical datasets are provided during data center visits.
- Review results for disclosure risk and provide them once analysis is completed at data centers.
Researchers with approved applications must complete the following steps to prepare for data access and utilization.
Discuss the committee’s approval with the assigned RDC analyst and address any revision requests as needed.
Provide the RDC analyst with approved data dictionaries, public-use and non-NCHS data, descriptions of desired data linkages with intended final formats, and clearly defined derived variables, including arithmetic code or algorithms.
Complete the Confidentiality and Disclosure training and forms, then provide them to the RDC analyst. Additional forms, documents, and tools are available on the RDC Reference Materials page 19,20.
When invoiced, pay fees incurred by the request and applicable to data access at the intended location and frequency. Complete details are in Fees and Invoicing 21.
Amendments for project changes are possible but require additional approval prior to implementation. Common reasons for an amendment include, but are not limited to, changes in the research team, requests for additional variables, new methods, or requests for additional types of output 13. However, if the scope or research question changes, a new application is required instead.
Contact the RDC directly at rdca@cdc.gov for further guidance.
d. Accessing the Data and Publication Expectations
- After completing the steps in Part C, the RDC analyst will prepare the dataset. Once it is ready, schedule an appointment to access the data at the chosen RDC location. Schedule appointments at least one week in advance.
Different data centers have different access procedures and may incur additional fees depending on frequency of data access. Refer to the Data Distribution Centers section and associated links for additional guidance.
- Upon completion of analysis, submit an output request to the RDC analyst for review and approval. Full details about requirements and expectations are on the Output Policies and Procedures page 22.
NCHS does not guarantee that study-generated output will be released due to concerns related to, but not limited to, privacy and alignment with the approved application.
- When output is nearly complete, send it to the RDC analyst for review before submitting for publication or distribution. Full details about requirements and expectations are on the Publishing Guidelines page 23.
Data Distribution Centers
NCHS data is available at two types of data centers: the Census Bureau’s Federal Statistical Research Data Centers (FSRDC) and the NCHS Research Data Centers (RDCs). Additional details about preparing to access data at one of these sites can be found on the RDC Location of Access page or their respective subpages: FSRDC and NCHS RDC 13,24–26.
Submit an electronic copy of any notes or reference materials needed to the RDC analyst prior to visiting a center. Hard copies of these materials are not allowed. Electronic communication devices, such as phones, pagers, and laptops, are also not permitted in the RDC.
Different software products or add-ons can be requested, though not all requests will be approved. Be aware that requesting additional software accommodations may delay project approval. Contact the specific RDC where data will be accessed for further guidance.
Federal Statistical Research Data Centers
Researchers must be affiliated with a university or agency to qualify for FSRDC access. They must also meet physical and information security requirements, including obtaining Census Bureau Special Sworn Status (SSS) and passing a background investigation. Non-U.S. citizens are generally encouraged to use FSRDCs.
Researchers are assigned both an NCHS RDC analyst and an FSRDC administrator from the location they intend to visit. The NCHS RDC analyst roles were described previously under Part C of the Step-by-Step Guide. The FSRDC (Census RDC) administrator will:
- Be available to answer questions pertaining to SSS, access and entry to a FSRDC location, software availability, and additional Census fees.
- They may be available to help develop the application.
- Transfer output to the NCHS after researchers complete their analysis at a data center. The RDC analyst will review the output for disclosure risk and provide researchers with the results.
If accessing an FSRDC, the data must be transferred from NCHS after completing all requirements outlined by the assigned RDC analyst seven days prior to the intended visit. These additional steps incur extra costs beyond the NCHS RDC data access fee and may delay data access.
- In-person: Census Bureau facilities at partner institutions.
- Remote access: A secure Virtual Desktop Interface (VDI) may be available.
- Software Available: Anaconda, Gurobi, Intel Composer, Knittro, MADD, Mathematica, NATLAB, OpenGeoda, R and Rstudio, SAS, Stat/Transfer, Stata, Stata-MP, SUDAAN, and Tomlab.
NCHS Research Data Centers
- In-person: Facilities located in Hyattsville, MD, Atlanta, GA, and Rockville, MD require appointments scheduled at least one week in advance.
- Remote access: Not available due to computers being disconnected from the internet.
- Software Available: Microsoft Office products, R, SAS, Stata, Python, SPSS (v. 19), and SAS-callable SUDAAN.
Required Documentation
When accessing the data, researchers must provide the following documentation in addition to an approved application:
- Proof of identification, such as a REAL ID
- Curriculum vitae (CV) for each applicant
- For student projects, an agreement form completed by the student’s advisor 27
- Permission to use proprietary data
- Table shells for requested output
- Data dictionary listing all necessary variables (restricted-use, public-use, and external) for the research project (refer to agency dataset data dictionaries for available variables)
Relevant Links
Vital Statistics Online Data Portal: Find links to all publicly available data and their user guides, categorized by U.S. state and city data and U.S. territories data 4.
CDC’s Wide-ranging ONline Data for Epidemiologic Research (WONDER): Use the CDC WONDER API to select and query custom combinations of some publicly available NVSS data 28.
Restricted-Use Vital Statistics Data: Find links to descriptions of data files and records available in the restricted dataset, along with the conditions for using these datasets 3.
Where to Write for Vital Records: Find links to information on how to directly access individual state, city, and territory vital statistics 29.
Data Release Policy for Vital Statistics Micro-data Files: Find the current NCHS/Division of Vital Statistics (DVS) policy on micro-data for births, deaths, fetal deaths, linked birth/infant death, and matched multiple births.
NCHS Data User Agreement: Find the data use limitations and expectations set by NCHS for using their publicly available data 30.
Confidentiality and Disclosure: Find study participant confidentiality training, documentation, forms, and manual 20. These trainings and forms must be completed following approval of the study proposal by the research committee.
Application Process: Find the complete step-by-step guide for applying for restricted-use data from the RDC 13. Key information is summarized under the Gaining Access - Restricted Access tab.
RDC Reference Materials: Find restricted-use data proposals, example applications, confidentiality and disclosure documents, rules, policies, and data protection toolkits 19.
Model State Vital Statistics Act and Regulations: Find some of the guidelines provided to states, cities, and territories to standardize the collection, recording, and reporting of vital statistics 31.
NCHS Data Visualization Gallery: Find links to interactive data visualization dashboards created using NVSS data 32.
What’s New: Find a listing of previously published NVSS reports, data release notices, and training materials. Note that some of these resources are intended for a broader audience beyond researchers using NVSS data in their studies 33.
Publications
This section presents a selection of PubMed articles that utilize the dataset and are authored by individuals affiliated with the Yale University. These articles are provided to inspire researchers and students to use the data in their own work.
-
Years of life lost due to central nervous system tumor subtypes in the United States.
Jakob V E Gerstl, Mackenzie Price, Joshua D Bernstock, Carol Kruchko, Lennard Spanehl, Paramesh V Karandikar, Jill S Barnholtz-Sloan, Timothy R Smith, Elizabeth B Claus, Quinn T Ostrom
Neuro-oncology 2025 Jun 15 pii: noaf142. doi: 10.1093/neuonc/noaf142
PMID: 40517297 -
Sex-selective abortion bans and the birth outcomes of Asian immigrants.
Emma Zang, Keitaro Okura, Melissa Tian
Social science & medicine (1982) 2025 Jul 22 doi: 10.1016/j.socscimed.2025.118442
PMID: 40752241 -
Association of Prepregnancy Cardiometabolic Factors With Gestational Diabetes Among Asian Populations in the United States.
Theresa Boyer, Christine Hsueh, Kevin Sun, Yaa Adoma Kwapong, Arthur Jason Vaught, Justin Echouffo Tcheugui, Elizabeth Selvin, Chiadi E Ndumele, Allison G Hays, Erin D Michos, Josef Coresh, Anum S Minhas
JACC. Asia 2024 Sep 24 doi: 10.1016/j.jacasi.2024.07.010
PMID: 39619403 -
Interaction Effects of Maternal Sexually Transmitted Infections with Prenatal Care Utilization Status on Preterm Birth and Low Birthweight: U.S. National Data.
Anthony J Kondracki, Wei Li, Zoran Bursac, Manouchehr Mokhtari, Bonzo Reddick, Jennifer L Barkin
Journal of clinical medicine 2022 Sep 1 doi: 10.3390/jcm11175184
PMID: 36079115 -
Disparities in air pollution attributable mortality in the US population by race/ethnicity and sociodemographic factors.
Pascal Geldsetzer, Daniel Fridljand, Mathew V Kiang, Eran Bendavid, Sam Heft-Neal, Marshall Burke, Alexander H Thieme, Tarik Benmarhnia
Nature medicine 2024 Jul 1 doi: 10.1038/s41591-024-03117-0
PMID: 38951636