Updated: November 5th, 2025
Overview
UK Biobank provides an ongoing clinical study biobank offering the most comprehensive dataset of biological, health, and lifestyle information from half a million participants since initial recruitment in 2006 1,5. Participants were recruited from 22 centers in Scotland, England, and Wales, with over 86% living in urban areas. Since recruitment from 2006 to 2010, these participants have consistently provided data on health records, physical measurements, lifestyle and environment, questionnaires, and biospecimens 1,6.
The UK Biobank continually enhances its research value by integrating biomedical data with external sources, collecting additional data points, and generating new data from biospecimens. They have introduced new research initiatives, including lifestyle questionnaires, wearable technology, and one of the world’s largest sleep surveys and whole-body imaging projects 2–4.
Participants were aged between 40 and 69 years and living in the UK when they joined the study between 2006 and 2010, with half being female. They represent various socioeconomic backgrounds, with more than 40,000 born outside the UK. Participants tend to be wealthier and healthier than the national average, reflecting a common ‘healthy volunteer bias’ 5–7. Despite this, the sufficient number of participants across various risk levels allows the findings to be broadly generalizable to the UK. 94.6% of participants are of white ethnicity, reflecting the national population at the time of recruitment (94.5% in the 2001 UK Census and 91.3% in 2011). Consequently, the data does not effectively represent disease risk across different ethnic groups 6.
The types of data collected include 8:
Demographic, Lifestyle, and Self-Reported Health Data: Basic demographics and socioeconomic status; family characteristics like illnesses of parents and siblings; lifestyle habits such as physical activity and diet; sociodemographic factors like education and employment; and various medical conditions including eyesight and mental health 9.
Imaging: Magnetic Resonance Imaging (MRI) scans of the brain, heart, and abdomen, full-body bone density scans (DXA/DEXA) scans, carotid ultrasound images, Optical Coherence Tomography (OCT) scans, and COVID-19 repeat scans 10.
Biomarker: 251 metabolic biomarkers, 3,000 proteins using OLINK’s proteomic assays, infectious disease antigens, 34 components in blood and urine, and blood counts including examples such as basophils, eosinophils, neutrophils, red blood cells, white blood cells, platelets, and haemoglobin 11.
Genetic: Around 850,000 genetic variants directly measured, more than 90 million variants predicted using statistical methods, whole exomes and genomes, and telomere length 12.
Health Records: Coded hospital data for diagnoses and procedures, coded general practitioner (GP) data containing codes about diagnoses, prescriptions, and referrals (excluding confidential notes or letters), cancer diagnoses from national cancer registries, deaths data, and algorithmically classified outcomes based on qualifying diagnostic and/or procedural codes for conditions such as asthma, COPD, dementia, myocardial infarction, and stroke 13.
Physical Measurements: Collected during the first “baseline” visit: participants’ height, weight, waist and hip circumference, lung capacity, body composition (muscle and fat mass), hand grip strength, heel bone density, blood pressure, arterial stiffness, vision, hearing, touchscreen tests for memory, fitness level and activity monitored for one week, and 12-lead electrocardiogram (ECG) 14.
Questionnaire Results: Some participants completed detailed questionnaires covering topics such as diet (e.g., food preferences and a 24-hour food recall), cognitive function, occupational history, mental health, digestive health, pain, and overall health and wellbeing 15.
Environmental Data: Air pollution near participants’ residences, noise pollution, greenspace in participants’ local areas, the nearby built environment and traffic intensity, the distance of their homes to the coast, and tap water minerals 16.
Gaining Access
Do I Qualify?
UK Biobank data are made available to eligible researchers from academic, charity, government, and commercial organizations worldwide for health-related research that serves the public interest. Any research using UK Biobank data must aim to positively impact public health and wellbeing while adhering to stringent ethical standards. The research must not cause harm, discrimination, mistreatment, or marginalization of any social groups, thus supporting inclusivity and societal benefit 17.
To be eligible to access the data, researchers must demonstrate a proven track record of legitimate health-related research. They must also be affiliated with a recognized research organization and operate from a country that complies with international regulations and is not subject to UK, US, or EU sanctions, as outlined in the UK Biobank International Sanctions Policy 18,19. Additionally, the UK Biobank no longer accepts applications from insurance companies, as specified in the Access to UK Biobank Data section under “Controlling Access” 17.
UK Biobank ensures compliance with these eligibility requirements by conducting thorough background checks on researchers applying for data access. This is done by trained personnel who verify the legitimacy of the applicants and their research credentials 17.
How Is My Application Assessed?
The application process will involve the UK Biobank Scientific Team and Access Committee (AC), a sub-committee of the UK Biobank Board 20,21. The scientific team will evaluate the information provided in the data request application to determine if the application meets the required access criteria 22.
The AC, which meets on a quarterly basis, is responsible for making key access decisions, notably those regarding the use of depletable samples, recontact, or potentially contentious research. The AC will assess the application for 22:
- The likelihood of the proposed research receiving approval
- The proposed research’s compliance with the required access criteria (including legal and ethical standards)
- The availability of the requisite data and/or samples for the proposed research
- The scientific justification for the amount of depletable sample required
- The cost associated with providing the requested data and/or samples
There are four possible outcomes 21:
The UK Biobank Scientific Team approves the application and presents it to the Access Sub-Committee, which may raise any queries.
The application is sent back to the Applicant Principal Investigator (PI) for revisions.
The UK Biobank Scientific Team recommends the application for denial and escalates it to the Access Sub-Committee, which will review and discuss it. The Sub-Committee may approve or decline the application, providing reasons and suggestions for a successful future application if applicable.
The UK Biobank Scientific Team forwards the application to the Access Sub-Committee for detailed consideration and approval due to unusual, contentious, ethical-legal, or other novel issues.
Additional details describing how applications are evaluated, and the responsibilities involved, can be found in the Access Procedures document, available under the section “Key Documents.” Specifically, refer to Section C10, “Roles of the Parties in the Application Review Process” 19,21.
Most of the details summarized in the remaining sections about “Gaining Access” were sourced from the Access Procedures document 19,21. If any information was supplemented or sourced from other resources, it is cited accordingly.
Typical Timeline
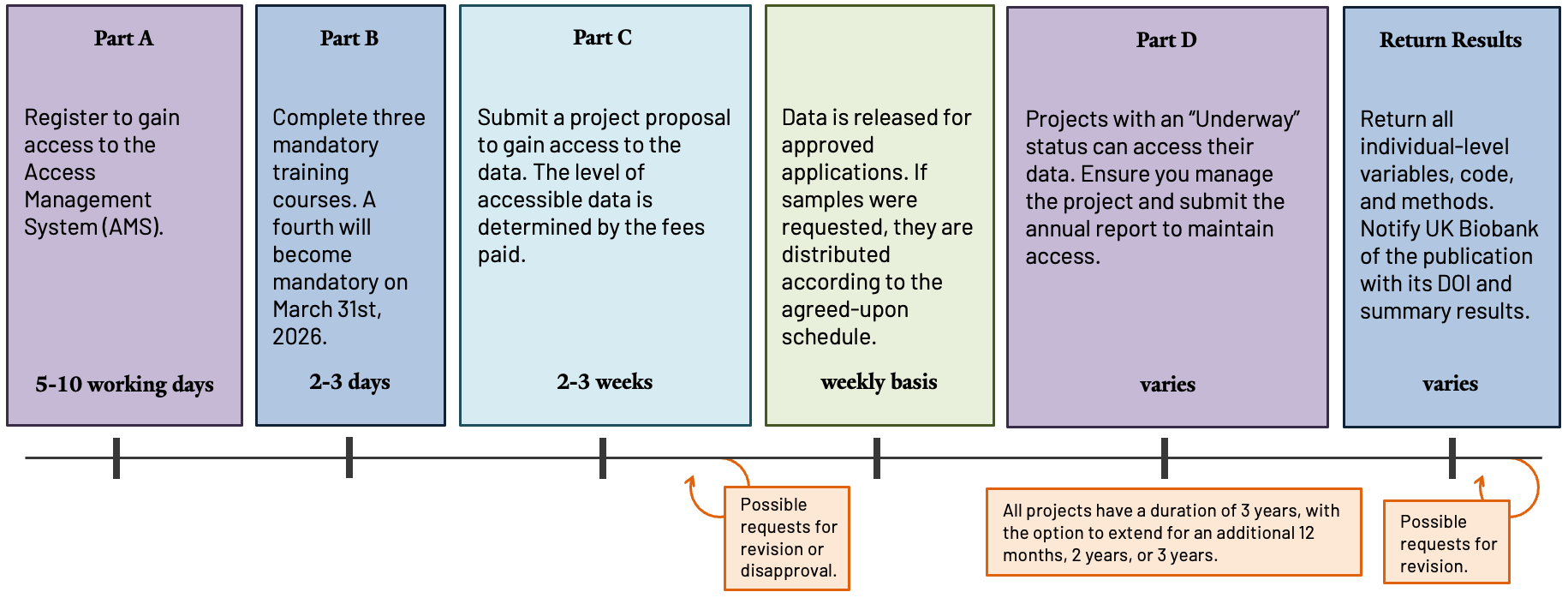
Step-by-Step Guide
All researchers must register, complete training modules, and sign user agreements in the UK Biobank Access Management System (AMS) before they can request data 25. The AMS allows you to manage your portfolio information and check which outstanding trainings need to be completed to gain access.
Data is accessed via the DNAnexus UK Biobank cloud computing portal, Research Analysis Platform (UKB-RAP), provided in conjunction with Amazon Web Services (AWS). UK Biobank offers the opportunity to request data downloads, which are individually assessed and approved by the UK Biobank Access and Scientific Teams. These teams make a recommendation to the Access Committee, which then reviews the application 26. More details on this process can be found below.
It is recommended to use Google Chrome to access the AMS due to identified compatibility issues with certain browsers, such as Firefox and Safari.
a. Register for Access in the Access Management System (AMS) 27,28
If your institution is not approved, you will not be able to complete AMS registration until it gains approval to access UK Biobank data.
Some of these answers, along with details of the research the user participates in, are made publicly available on the UK Biobank website. You can find a list of all ongoing, approved projects on their Existing Projects page 29.
Register for an AMS account for free using your institution’s email address.
Verify your new account using the 24-hour verification link sent to your registration email.
To complete the registration process, log in to the UK Biobank AMS system using your recently verified credentials.
You will then see a message window that prompts you to complete your registration by providing additional details about you. The mandatory fields include:
- Your full name, phone number, and date of birth
- An up-to-date resume/CV in English confirming your current position and institution, OR a link to a personal profile page on your institution’s website
- PubMed reference numbers for up to 5 peer-reviewed publications where you are listed as an author (if available)
- Details of any complaints raised against you within the past 3 years (if applicable)
- Name of the primary institution you are affiliated with that has approved access to UK Biobank data
- Your email address with this institution and used for registration
- Your position at this institution
- List other institutions with which you plan to use the UK Biobank Resource (if applicable)
- The registration team will review your application and send a message within the AMS portal. They may contact you to request further details.
b. Complete the Training Modules 25
The Home page of your AMS portal will display notices about mandatory training along with any announcements or important information. Outstanding training module requests can be found on the Profile page in the “Training” section. Additionally, you can find previously uploaded Medical Research Council (MRC) certificates there.
You must annually complete the MRC course “Research, General Data Protection Regulation (GDPR) & Confidentiality” and upload the passing certificate 27. You may also be asked to recomplete the two introductory courses and their accompanying multiple-choice tests.
Complete the MRC course “Research, General Data Protection Regulation (GDPR) & Confidentiality”, which consists of 10 short video. If this is your first time logging into the MRC Centre for Research Policy Learning Management System, you will need to first create and verify a new account.
Take the MRC course 10-question multiple-choice quiz. You must pass with a score of 70% or higher for the certificate to be accepted by the Access team.
Save a PDF copy of the passing completion certificate and upload it in the “Upload MRC Certificate” section of your AMS Profile page.
Complete the course video “Introduction to using UK Biobank” 30.
Complete the course video “Introduction to the UK Biobank Research Analysis Platform (RAP)” 31.
Take the two multiple-choice tests, found in the “Training” section of your AMS Profile page. You must pass with a score of 80% or higher on both tests to complete the process.
The three listed courses are mandatory to gain access. Additionally, UK Biobank has recently released the “Code Repository” course, which includes its own multiple-choice test. This course is planned to become mandatory starting March 31\(^{st}\), 2026 32.
c. Apply to Receive Data 17,28
Once registration is completed, an “Applications” page link will appear in the sidebar 22. There are two options when requesting access to data: data only or data and biospecimens. Both application types have distinct features but follow similar review processes, which are summarized below. Researchers can find specific details about the application contents in their AMS portals.
Some of these answers, along with details of the research the user participates in, are made publicly available on the UK Biobank website. You can find a list of all ongoing, approved projects on their Existing Projects page 29.
- The applicant PI must complete and submit an application for either data only or data and biospecimens. Some of the fields in the application might include:
- A summary of the research you intend to conduct in the project, including the research questions, objectives, and scientific rationale (displayed publicly)
- Keywords describing the research
- Confirmations that the research will be used to perform health-related research and ultimately aims to improve public health
- The type and size of the data requested
- Confirmation if your research requires biospecimens, and if recontacting participants is needed
- Description of the methods
- A statement if the study will result in the generation of new data
List all project collaborators along with their contact details. Additionally, you must include contact information for a signatory authorized to sign the Material Transfer Agreement (MTA) on behalf of each institution collaborators are from.
Indicate the tier of data you are requesting, as each tier comes with different associated costs. For more information on the fees, visit the biobank’s Fee Structure page 33.
Submit the form for review by the UK Biobank Scientific Team and AC, which meets quarterly. Refer to the previous section titled “How Is My Application Assessed?” for more details about this process.
Approved applications will require the execution of the MTA and payment of access charges within 90 days of receiving the notice. Only then will the data and/or samples be released to the Applicant PI 22.
Data is usually released weekly, whereas samples are distributed following a predetermined schedule 22.
If your application was rejected, you can apply for reconsideration, as detailed in Section C4.4-5 of “Application Reviewed” found in the Access Procedures document available under the section “Key Documents” 19,21.
d. Accessing the Data
Approved applications with a signed MTA and paid fees will have a project status of “Underway” on AMS, enabling associated researchers to access the data in the UKB-RAP 25.
Create a DNAnexus account and associate it with your UK Biobank AMS account: Connect Your Account to UK Biobank.
There is no additional charge by UK Biobank to access the RAP. However, certain activities within the RAP will incur costs payable to the platform provider, DNAnexus. These include fees for data storage (both uploaded and derived data), computing and analysis (based on instance type), and data transfer charges. To assist researchers in managing these costs, UK Biobank provides an initial £40 of credit towards these expenses upon joining UKB-RAP 33.
In addition to signing agreements with UK Biobank, researchers using the RAP will also need to accept certain terms and conditions to use DNAnexus. These terms can be found on their Subscription Terms of Service page 34.
All approved projects will have a minimum of 3 years to conduct their research, with the option to apply for extensions of 12 months, 2 years, or 3 years if necessary 24.
To maintain access for the duration of a research project, the researcher must do the following. For more details about how UK Biobank expects PIs and researchers to manage ongoing projects, refer to the Step 4: Manage your project list of articles 19,35.
All researchers must keep their information in the AMS updated and ensure their training is current 19.
Applicant PIs and Lead Investigators at Collaborator Institutions must annually submit a project report, confirming project affiliates, compliance with the MTA, security expectations, and providing project updates along with an outline of activities planned for the next 12 months. For more details, refer to Submitting an annual report 36.
- Researchers are expected to promptly notify the UK Biobank when their project has been completed, regardless of the remaining time allowed to complete the project.
Return of Results and Publication Expectations
The UK Biobank has specific expectations and procedures for conducting research using their data, which include the handling of generated results, the code used to analyze the data, and any form of publication of findings. These procedures are outlined below, with some of them being critical both to maintain access to the data and to fulfill the UK Biobank data use agreements.
Researchers are encouraged to review the Guidance on Researcher Responsibilities and Step 5: Return your results pages, as well as Section B8 “Publication of findings, summary datasets and return of results” in the Access Procedures document, available under the “Key Documents” section, to fully understand these expectations 19,21,37,38.
The UK Biobank provides specific guidelines for acknowledgments in publications, reports, and press releases. These can be reviewed in the previously linked articles and on their Communications guidelines page 39. For example, you must include the following statement in the Acknowledgments section of your paper or wherever possible in reference search tools 40:
“This research has been conducted using the UK Biobank Resource under Application Number xxxxx.”
Summary of the Return of Results 38
Researchers are required to return all key individual-level variables derived from bulk data, as well as any related research outputs, within 6 months following the publication of their results or 12 months after the conclusion of their project, whichever occurs first. This submission must include a comprehensive data dictionary, any well-annotated code or methods used, and the corresponding DOI.
This transfer can be accomplished using the RAP system to directly send the generated information to UK Biobank. For detailed guidance on what is expected when returning results, please refer to the full guide available on the Returning your results to UK Biobank webpage 38.
Researchers are not obligated, but may choose to share their code, example notebooks, or tools to be freely utilized by other researchers.
The UK Biobank offers supporting materials to assist researchers in appropriately using Git and GitHub with sensitive data. Recently, they released the “Code Repository” course in AMS, which includes a multiple-choice test. Additionally, they have articles provide guidance on using Git and GitHub with their data, including links to instructional content and online courses about managing code-based projects. These resources are available on the Using Code Repositories page 32,41.
Researchers are also encouraged to review the UK Biobank GitHub for code support, including tools like the UKB-Git-Audit-Tool, which scans the project’s Git commit history for any potentially confidential information 42.
The DSDE team organizes freely accessible workshops featuring real-world, hands-on examples that participants are encouraged to explore or attend. Some of our workshops, including “Getting Started with Git and GitHub,” are being adapted for asynchronous learning on our Book of Workshops webpage 43. You can find information on upcoming workshops on our events page, and past workshop materials are available on the Book of Workshops.
Summary of Publication Expectations 37,40
Researchers are expected to publish a commensurate level of findings within the first 3 years of receiving approval for their research project. If this is not possible, the Applicant PI must provide a reasonable explanation, such as in the annual project report, detailing why this is not feasible and providing an estimation of when the findings can be published.
Researchers are expected to publish their findings in an academic journal or via an open-source publication site as soon as possible after the project’s completion, and no later than 6 months afterward.
UK Biobank supports publishing summary data tables, scans, and images alongside main articles but notes that these should not include any Participant Level Data or features that might be identifiable, such as reconstructions of the head and skull.
You are encouraged to contact UK Biobank regarding any concerns about using summary data tables, scans, and images at access@ukbiobank.ac.uk prior to submitting the publication.
- UK Biobank does not require the PI to obtain approval before publishing. However, the Applicant PI must provide a copy of any report on their project’s findings and/or any press release to UK Biobank at least 2 weeks before the first public presentation or publication (e.g., meeting abstract, online report, pre-print server, and journal).
This notification can be fulfilled by submitting a request or submitting the materials through the AMS.
- For press releases, especially those likely to provoke controversy or attract significant public attention, the Applicant PI must follow the Communications guidelines and share a copy of the release with the UK Biobank communications team at communications@ukbiobank.ac.uk 39.
- To use UK Biobank scans or images in a publication, you will need to submit a request with the image and Project ID. Any approved images should include the following acknowledgment:
Reproduced by kind permission of UK Biobank ©
- You must acknowledge any data linked to in your analysis. For example:
“this work uses data provided by patients and collected by the NHS as part of their care and support.”
Requesting Downloading Data
In the past, researchers had to download requested data and agree to delete it upon completing their study. With the introduction of the UK Biobank RAP, researchers are now encouraged to access all data for their research directly on the platform. However, there may be extenuating circumstances that the UKB-RAP cannot accommodate. For example, researchers might:
- Require tools or specialized software not yet integrated with the UKB-RAP
- Face prohibitive costs for specific cloud-based analyses
- Require substantial compute power (GPUs) that exceeds the current capacity of the UKB-RAP
To ensure accessibility for health-related research, the UK Biobank AC will assess applications to download certain types of data. You can find the full exemption policy here: UK Biobank exemptions from the UKB-RAP by default policy 26. If approved, researchers will then be allowed to temporarily download the data.
All projects with approval to temporarily download data will be subject to Tier 3 fees 33.
Certain large datasets, including the Whole Genome Sequence data and the Whole Exome Sequence data, will only be available through the RAP. Researchers are expected not to attempt to circumvent the requirement to access these datasets solely via the RAP. For example, researchers must not make minor modifications (such as adding a ‘0’ to the data content) and then treat the data as derived to qualify for download.
For more information about UK Biobank’s policies and common FAQs on downloading data, please refer to the Downloading data page 44.
Financial Support 45
UK Biobank offers reduced access fees for researchers from Low and Low-Middle Income Countries and for postgraduate students. With the reduced fee, you will be able to access all datasets through the RAP but will not have permission to request waivers to download any data 33.
For students seeking reduced fees, you will need to meet the following criteria:
The application must be submitted by a student or their supervisor exclusively for the purpose of completing a postgraduate student project (e.g., MSc, PhD, or equivalent). The student must be the lead author of any resulting publications.
The application is not allowed to be used for other research purposes, nor can it be utilized for multiple student (or other) projects.
Collaborators must have a specific and relevant role in the student’s research project and may only be included at the discretion of UK Biobank.
You can learn more about applying for Financial Support on the UK Biobank page dedicated to this topic.
Relevant Links
Get started with UK Biobank: Find articles about gaining access to UK Biobank data, links to policies, an FAQ for common problems, a listing of mandatory trainings, and articles on how to manage your active projects and share results 46.
UK Biobank DNAnexus and About the Research Analysis Platform: Find more about the DNAnexus RAP platform developed for UK Biobank, as well as the database documentation. This includes the Tools library, which contains listings of all the analytical tools available to researchers 47–49.
Types of data: Explore a breakdown of the various data categories provided, with links to additional documentation and the relevant Showcase Data Browser pages 8.
Showcase Data Browser: Find data field descriptions, glossaries, meta-information linking schemas, high-level, de-identified summaries of data contents for each data field, and instructions on navigating the biobank system, along with technical information for researchers interested in using the data 50,51.
Use Our Data: Find links to relevant articles and webpages that guide researchers through data access applications, training and support options, financing, and facilitated collaboration 52.
Protecting the Data: Find information about the data security measures taken to ensure participant confidentiality and monitor the appropriate use of their data for research. Additionally, find a link to the UK Biobank data de-identification protocol 53.
Existing projects, Publications, and Research Stories: Find a list of ongoing and completed research projects using UK Biobank data, as well as articles highlighting impactful research conducted with UK Biobank data from around the world 29,54,55.
UK Biobank YouTube: Find informative videos from UK Biobank staff and interviews with researchers discussing their findings using UK Biobank data. Subjects covered include training modules, conference recordings, and promotions of recent news or upcoming features on the platform 56.
UK Biobank GitHub: Find coded examples using the UK Biobank Research Analysis Platform (UKB-RAP), auditing tools to ensure data security in Git repositories, and example workflows for genomic and image analysis 42,57.
Our Future: Find information about upcoming expansion projects, outreach, infrastructure improvements, funding, and financial support 2.
Researcher Events: Find events specifically geared towards the research community 58.
News: Find the latest stories from UK Biobank, including news about data releases, new activities to enhance the data, and updates about people and funding. At the time of this writing, the biobank was celebrating the upcoming release of the largest collections of whole body imaging and sleep survey data 3,4,59.
Publications
This section presents a selection of PubMed articles that utilize the dataset and are authored by individuals affiliated with the Yale University. These articles are provided to inspire researchers and students to use the data in their own work.
-
Observational and Genetic Analyses of Traumatic Experiences and Endometriosis.
Dora Koller, Solveig Løkhammer, Oksana Goroshchuk, Veronika Denner, Brendan Stiltner, Marina Mitjans, Jun He, Hugh S Taylor, Rebecca B Lawn, Karestan C Koenen, Renato Polimanti
JAMA psychiatry doi: 10.1001/jamapsychiatry.2024.4694
PMID: 39908042 -
Association of Unhealthy Lifestyle and Childhood Adversity With Acceleration of Aging Among UK Biobank Participants.
Gan Yang, Xingqi Cao, Xueqin Li, Jingyun Zhang, Chao Ma, Ning Zhang, Qingyun Lu, Eileen M Crimmins, Thomas M Gill, Xi Chen, Zuyun Liu
JAMA network open 2022 Sep 1 doi: 10.1001/jamanetworkopen.2022.30690
PMID: 36066889 -
Artificial Intelligence-Enabled Prediction of Heart Failure Risk From Single-Lead Electrocardiograms.
Lovedeep S Dhingra, Arya Aminorroaya, Aline F Pedroso, Akshay Khunte, Veer Sangha, Daniel McIntyre, Clara K Chow, Folkert W Asselbergs, Luisa C C Brant, Sandhi M Barreto, Antonio Luiz P Ribeiro, Harlan M Krumholz, Evangelos K Oikonomou, Rohan Khera
JAMA cardiology doi: 10.1001/jamacardio.2025.0492
PMID: 40238120 -
Autoimmune Diseases and Risk of Non-Hodgkin Lymphoma: A Mendelian Randomisation Study.
Xiaoting Shi, Joshua D Wallach, Xiaomei Ma, Tormod Rogne
Cancer medicine doi: 10.1002/cam4.70327
PMID: 39506244 -
Identification of hypertrophic cardiomyopathy on electrocardiographic images with deep learning.
Veer Sangha, Lovedeep Singh Dhingra, Arya Aminorroaya, Philip M Croon, Nikhil V Sikand, Sounok Sen, Matthew W Martinez, Martin S Maron, Harlan M Krumholz, Folkert W Asselbergs, Evangelos K Oikonomou, Rohan Khera
Nature cardiovascular research 2025 Jul 22 doi: 10.1038/s44161-025-00685-3
PMID: 40696040 -
Association of Childhood Adversity With Frailty and the Mediating Role of Unhealthy Lifestyle: A Lifespan Analysis.
Gan Yang, Xingqi Cao, Jie Yu, Xueqin Li, Liming Zhang, Jingyun Zhang, Chao Ma, Ning Zhang, Qingyun Lu, Chenkai Wu, Xi Chen, Emiel O Hoogendijk, Thomas M Gill, Zuyun Liu
The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 2023 Sep 1 doi: 10.1016/j.jagp.2023.08.015
PMID: 37770350